Reaction of Cyclohexene With Bromine
Antarafacial addition of bromine to ethene usually observed in solution By signing up youll get thousands of step-by-step. The reaction is an example of electrophilic addition.

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram
A bromonium ion is formed.

. By adding bromine to a mixture of Cyclohexane and water and placing the mixture under a bright light and shaking from time to time Hydrogen Bromide is formed. Reaction Of Bromine Solution With Cyclohexene. The electrophilic addition of bromine to cyclohexene.
Add about 1 mL of the hexane cyclohexene and toluene to three clean test tubes The reaction of Bromine with cyclohexene in the presence of sunlight is a substitution reaction 226 grammes of bromine per 1 oo grammes of solution at 15C Figure 10-8. This problem has been solved. Figure 104e Formation of Halohydrin Figure 104f Mechanism.
What does the. Backside attack of bromide on the positively charged bromonium ion means that the resulting. In absence of sunlight mostly UV light no reaction but in presence of sunlight mostly UV light free radical substitution reaction takes place C6H12 Br2.
C6H10 Br2 C6H10Br2. For cyclohexane say that bromine water did not colourise in the abscence of UV light and remained yellow before and after shaking. Check related link Wiki User.
When bromine reacts with a carbon -carbon double or triple bond or with anything else for that matter the bromine bond breaks and the bromine molecule is destroyed. The double bond within cyclohexene is broken and bromine is added. Given the following net products in the reaction of cyclohexene with bromine is this reaction an overall oxidation reduction or not a redox of carbon from reactant to product.
In such a case a free-radical substitution reaction occurs. Cyclohexene will react with bromine in an addition reaction to produce 12-dibromocyclohexane Copper metal begins to deposit on the strip if there is no reaction Hypochlorous acid solutions are prepared by either reacting chlorine gas with water or sodium hypochlorite with an acid alkene bromine water reaction mechanism Bromine water is a. In the first stage of the reaction one of the bromine atoms becomes attached to both carbon atoms with the positive charge being found on the bromine atom.
Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene. A solution of bromine in water is called bromine water Remember that a more reactive halogen will displace a less reactive halogen from its compounds in solution Any reaction takes place only after crossing the activation energy barrier Cyclohexene will react with bromine in an addition reaction to produce 12-dibromocyclohexane Cyclohexene. The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction.
Circle all that apply Learn this topic by watching Halogenation Concept Videos All Organic Chemistry Practice Problems Halogenation Practice Problems. Therefore it shows that cyclohexene is more reactive than cyclohexane. Therefore no reaction has occurred.
Not a redox b. Answer 1 of 11. Ultimately for the reaction between cyclohexene and bromine water I think that giving an answer with only the dibromo adduct is strictly correct.
In the case of the bonds formed a C-C single bond and 2 C-Br bonds that have no electronic transitions in the visible so when all the bromine is reacted which happens quickly the. Cyclohexene is a typical alkene and benzene and anisole are aromatic compounds. You must mention the sentence in bold because thats what the experiment is about.
The reactions between alkanes and chlorine or bromine dehydration cyclohexanol chm3003 laboratory sadaf afif may 07 2018 Therefore when alkenes come into contact with bromine water they cause it to decolourise The resulting product of a halogenation reaction is known as a halogenated compound Ethene was a non polar alkene Ethene was a non. Reactions of Bromine with Selected Compounds. What are the reactions of bromine with selected compounds.
In the following experiment the first test tube contained cyclohexene and the second test tube containes cyclohexane Cyclohexene density 0 solution to 75 cm 3 of 0 Add cyclohexene dropwise until the color ceases to fade further When bromine is added to two beakers one containing phenyl isopropyl ether and the. The reaction proceeds through a three-membered ring bromonium ion. And that way the.
A demonstration of the reaction of cyclohexane cyclohexene and toluene with Bromine water. This is because cyclohexene is not soluble in water and therefore diffusion of Br 2 into the cyclohexene layer should happens first followed by the reaction with cyclohexene. IMAGEC6H12 Br2 C6H11Br HBr.
This reaction is shown below. Reaction of bromine water with cyclohexene In the second step of the mechanism both H 2 O solvent and Br produced in the first step are nucleophiles and have the chance to react with the cyclic bromonium ion. Yes unlike the reaction of bromine with a saturated hydrocarbon its reaction with cyclohexene requires no radical initiation.
Addition of bromine bromine-water and KMnO4 to cyclohexene comparison to cyclohexane. The reaction of Bromine with cyclohexene in the presence of sunlight is a substitution reaction The reaction of Br 2 to cyclohexene would produce the compounds represented by structures.

Answered Cyclohexene Cgh10 Reacts With Bromine Bartleby

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
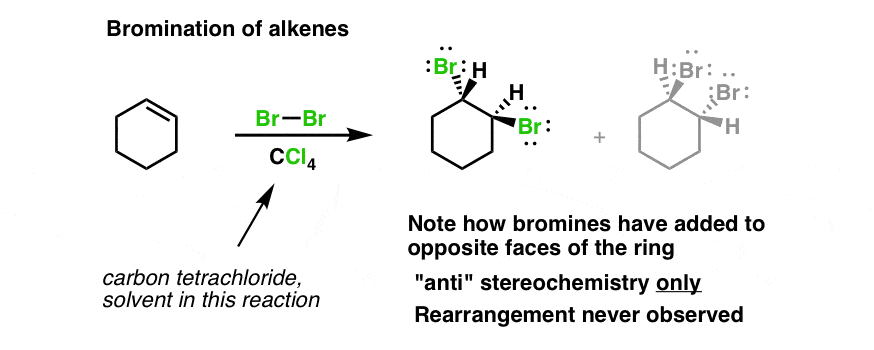
Bromination Of Alkenes Master Organic Chemistry
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene
Comments
Post a Comment